ILAE 2012 classification of electroclinical syndromes
and other epilepsies
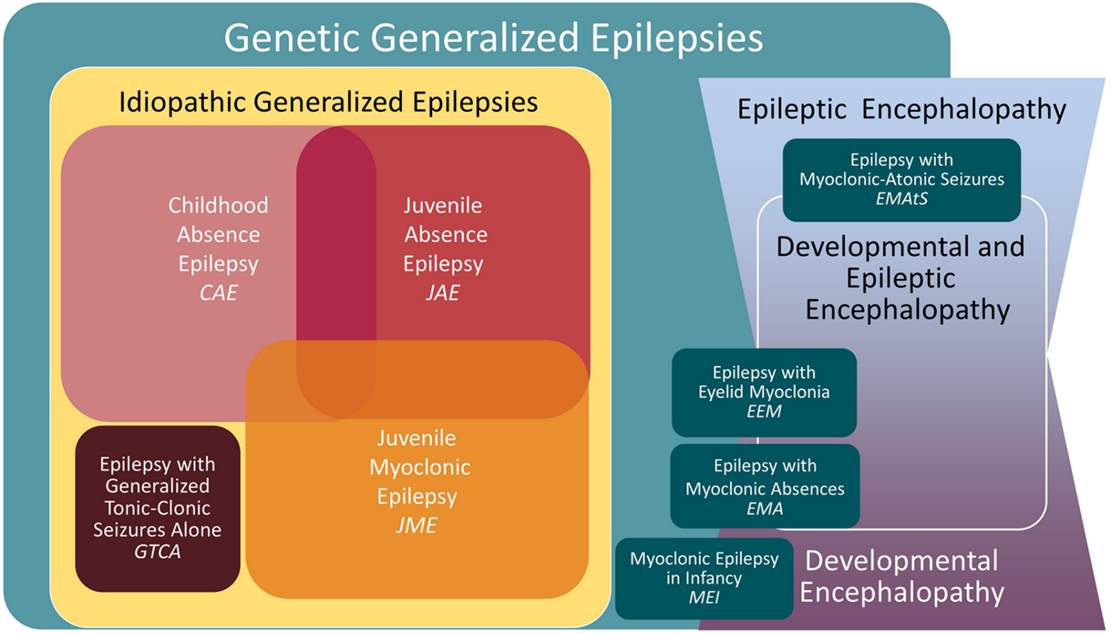
Specific Epilepsy Syndromes Genetic Generalised
Epilepsy
Idiopathic Generalised Epilepsies
Childhood Absence Epilepsy (CAE)
Juvenile Absence Epilepsy (JAE)
Early onset absence epilepsy (not accepted as official
category in ILAE)
Genetic Epilepsy with febrile seizures
Other Genetic Generalised Epilepsies
Epilepsy with Myoclonic Absence (EMA)
Epilepsy with eyelid myoclonia (EEM) (Jeavons syndrome)
GLUT 1 Deficiency (aetiological classification rather
than true syndrome)
Specific epilepsy syndromes Symptomatic
generalised epilepsy
Epilepsy with myoclonic atonic seizures (EMAtS) (Doose syndrome)
Progressive Myoclonic Epilepsies
Progressive, dementing conditions
Familial Adult Myoclonic Epilepsy (FAME)
Concept of Developmental/Epileptic Encephalopathies
Specific epilepsy syndromes Focal epilepsies
Sleep related hypermotor epilepsy (SHE)
Familial Mesial Temporal Lobe Epilepsy (FMTLE)
Familial Focal Epilepsy with Variable Foci (FFEVF)
Epilepsy with Auditory Features (EAF)
Self-Limited Focal Epilepsies (SeLFEs)
Childhood occipital visual epilepsy (COVE)
Photosensitive occipital lobe epilepsy (POLE)
Self-Limited Epilepsy with Centrotemporal Spikes
(SeLECTS)
Self-Limited Epilepsy with Autonomic Seizures (SeLAS)
Specific epilepsy syndromes Symptomatic/Lesional
Focal Epilepsies
Mesial Temporal Lobe Epilepsy with hippocampal sc
Autosomal Dominant Sleep-related Hypermotor Epilepsy
(ADSHE)
Autosomal Dominant Epilepsy with Auditory Features
Familial Focal Epilepsy with Variable Foci (FFEVF)
Familial mesial temporal lobe epilepsy
Epilepsy Syndromes
Classification
Individual seizures can be classified by type
o
Focal
o
Generalised
o
Unknown
Aetiology is next step in defining the epilepsy
o Three categories:
- Genetic
- Structural/Metabolic
- Unknown
Attempt to group seizures according to electro-clinical features into a particular group
o Electro-clinical syndromes
- Reasonably well defined, distinctive clinical disorder
o Constellations (Surgical syndromes)
- Groups of features with tend to occur together but not a clearly distinctive syndrome
Individual Seizure Types
Focal
o Aura (Sensory)
o Motor
o Autonomic
o With loss of awareness (dyscognitive)/ With preserved awareness
o May evolve to: Bilateral convulsive seizure
Generalised
o Tonic-clonic
o Absence
- Typical
- Atypical
- With special features:
Myoclonic absence
Eyelid myoclonia
o Clonic
o Atonic
o Myoclonic
- Myoclonic
- Myoclonic atonic
- Myoclonic tonic
Aetiology
Genetic
o The result of a known or presumed genetic mutation in which seizures are the core symptom of the disorder
Structural/Metabolic
o Structural (e.g. tumour or genetic malformation however not classified as genetic as there is another step interposed between the gene mutation and the epilepsy)
o
Unknown
Classification
- old
- Partial
/Localisation-related/ Focal seizures
- Idiopathic -
Benign childhood epilepsy with centro-temporal
spikes
- Symptomatic
- Temporal,
frontal, parietal, occipital lobe epilepsies
- Generalized
- Idiopathic
- Cryptogenic
or symptomatic - West, Lennox-Gastaut syndrome
OR
4 main types
|
Idiopathic Partial Epilepsy (20%) Mild,
focal onset seizures in children Usually
normal intellect Treatment
often minimal required e.g.
Rolandic epilepsy |
Symptomatic Partial Epilepsy (40%) Focal
onset seizures Lesions
of the cortex Usually
normal intellect Treatment
medication and surgery - First line Carbamazepine e.g.
TLE, AD frontal lobe epilepsy |
|
Idiopathic Generalised Epilepsy (30%) Generalised
seizures Inherited
ion channelopathies Normal
intellect Treatment
medication usually effective -
First line Valproate (e.g.
JME) |
Symptomatic (secondary) Generalised Epilepsy
(10%) Frequent,
severe, generalised seizures Intellectual
disability Treatment
hard to control e.g.
Lennox-Gastaut Syndrome |
ILAE 2021
classification of epilepsy syndromes
Classification
First determine if
the epilepsy fits into a specific electroclinical syndrome or constellation
If
not classify on the presence or absence of a structural or metabolic condition
(presumed cause) and then on the basis of the primary mode of seizure onset
(generalized vs. focal)
Electroclinical syndromes by age
Neonatal and infancy
Self-limited epilepsies
o Self-limited neonatal epilepsy
o Self-limited familial neonatal-infantile epilepsy
o Self limited infantile epilepsy
o GEFS+
o Myoclonic epilepsy in infancy
Developmental and epileptic encephalopathies (DEE)
o Early infantile developmental and epileptic encephalopathy (EIDEE) (prev. Ohtahara syndrome + early myoclonic epilepsy)
o Epilepsy in infancy with migrating focal seizures (EIMFS)
o Infantile epileptic spasms syndrome (IESS) (Prev. West Syndrome)
o Dravet syndrome (DS)
Aetiology specific syndromes
o GLUT1 DS
o Sturge-Weber syndrome
o Gelastic seizures with hypothalamic hamartoma
o Others ..
Childhood
Self limited epilepsies of childhood
o Self-Limited Epilepsy with Centro-Temporal Spikes (SeLECTS) (Benign Rolandic epilepsy)
o Self-Limited Epilepsy with Autonomic Seizures (SeLEAS) (Panayiotopoulos syndrome, early onset benign occipital epilepsy)
o Childhood Occipital Visual Epilepsy (COVE) (Late onset benign occipital epilepsy, Gastaut-type)
o Photosensitive Occipital Lobe Epilepsy (POLE) (Idiopathic photosensitive occipital lobe epilepsy)
Genetic generalised epilepsies
o Childhood absence epilepsy (CAE)
o Epilepsy with eyelid myoclonia (EEM) (Jeavons syndrome)
o Epilepsy with myoclonic absence (EMA) (Bureau and Tassinari syndrome)
DEEs
o
Lennox-Gastaut
syndrome (LGS)
o Epilepsy with Myoclonic Atonic Seizures (EMAtS) (Doose syndrome)
o Developmental/Epileptic Encephalopathy with Spike Wave Activation in Sleep (DEE or EE -SWAS)
- (Including subtype Landau-Kleffner syndrome)
o Febrile Infection Related Epilepsy Syndrome (FIRES)
o Hemiconvulsion-heiplegia Epilepsy Syndrome (HHE)
Onset at variable age
Generalised epilepsy syndromes
o Juvenile absence epilepsy (JAE)
o Juvenile myoclonic epilepsy (JME)
o Epilepsy with generalized tonicclonic seizures alone (GTCA)
Focal epilepsy syndromes (genetic)
o Self Limited (see childhood above)
- COVE
- POLE
o Familial mesial temporal lobe epilepsy (FMTLE)
o Epilepsy with auditory features (EAF)
o Sleep related hypermotor epilepsy (SHE) (Previously ADNFLE)
o Familal focal epilepsy with variable foci (FFEVF)
Focal and generalised
o Epilepsy with reading induced seizures (EwRIS)
Epilepsy with DE/EE
o FIRES (See above)
o Progressive myoclonic epilepsy
Aetiology-specific
o Mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS)
o Rasmussen Syndrome (RS)
Specific Epilepsy Syndromes
Genetic Generalised Epilepsy
Idiopathic Generalised Epilepsies
Childhood Absence Epilepsy (CAE)
4-8 years
Absence
GTCS 40%
Normal intellect
Underlying genes unknown
EEG
- 3Hz Spike-Wave
- Polyspike can be present
- Frontal predominant F3, F4, Fz and F7, F8
- Exacerbated by hyperventilation
- 80% of children with active CAE will have seizures induced by HV
Treatment
Ethosuximide (prevents absence but not GTCS)
VPA
LTG
Juvenile Absence Epilepsy (JAE)
Onset adolescence
Seizures
GTCS
Absence
Less frequent absence seizures compared to CAE - ~1-2/week
Each seizure is however longer ~20sec
EEG
Similar to JME (faster than CAE)
Treatment
VPA, LTG
Early onset absence epilepsy (not accepted as official category in ILAE)
Onset <4years
High frequency of developmental delay
10% due to GLUT1 deficiency
Juvenile Myoclonic Epilepsy (JME)
Early
adolescence (onset from 10-16years)
Polygenic cause
4% evolve from CAE
Generalized seizures absence seizures predominate at onset
Bilateral myoclonic jerks usually in the morning
o Consciousness usually preserved during jerks
o Single or repetitive
Most frequent in the morning after waking, provoked by sleep deprivation
EEG
Fast spike-wave discharges 4-5Hz
Polyspike
Small percentage exhibit photoparoxysmal response
Activated by hyperventilation and slow wave sleep
Suppressed during REM sleep
Epileptiform abnormality present in 90% on first EEG
Treatment
Benign, responds well to treatment (valproate), usually does not remit (although may reduce in later life)
Epilepsy with tonic-clonic seizures alone (GTCA)
GTCS with no other seizure types
Onset 10-25yrs (range 5-40yrs)
Seizure tendency usually lifelong and requires ongoing therapy
Genetic Epilepsy with febrile seizures
Febrile seizures extending beyond 6 years +/- development of afebrile seizures
Usually mild and resolves by adolescence
Some due to Na+ channel mutations
Some severe phenotypes
Other Genetic Generalised Epilepsies
Epilepsy with Myoclonic Absence (EMA)
Onset 2-12
EEG looks like CAE
Jerking tonic seizures
Absence seizures often atypical
Can be intellectually normal or have features of DEE
Epilepsy with eyelid myoclonia (EEM) (Jeavons syndrome)
Triad
o Frequent eyelid myoclonia
o +/- absence seizures
o Induced by eye closure and photic stimulation
1.2-2.7% of epilepsy
Onset peak 6-8 (range 2-14)
Female:male 2:1
Usually normal intellect (some exceptions)
Eyelid myoclonia can be associated with absence seizures with mildly impaired awareness
Can also be absence seizures separate to the eyelid myoclonia
GTCS occur in most patients, but are infrequent
GTCS can often be controlled with ASMs, however eyelid myoclonia often not controlled
Eyelid myoclonia may not be associated with EEG change in later life (i.e. a movement disorder)
GLUT 1 Deficiency (aetiological classification rather than true syndrome)
Can have variable phenotypical presentation
Can present as CAE phenotype
Absence seizures
Paroxysmal exercise induced dyskinesia (PED)
Lennox-Gastaut
Syndrome
Defined by:
o Multiple/mixed seizure types
o EEG showing slow spike and wave
o Impaired cognitive function
Epidemiology and aetiology
Children peak 2-5 years
M >F
Associated with a range of CNS diseases including: developmental abnormalities, trauma, infection, hypoxia etc.
Can evolve out of a previously recognised encephalopathy/cerebral injury OR occur de novo
Clinical
Seizure types
o Atypical absence
o Atonic seizures
o Tonic seizures
o Myoclonic seizures
Poor prognosis
EEG
Background
o Generalised and/or focal slow spike wave (1-2.5Hz) or polyspike
o Activated by sleep sometimes
o Brief bursts of rapid spikes in sleep
o Many patients manifest multifocal spikes or sharp waves
Absence seizures
o Slow spike-wave usually diffuse distribution
o Often less sharply demarcated onset and offset
Atonic seizures
o
Tonic seizures
o Brief runs of rapid spikes
Epilepsy with myoclonic atonic seizures (EMAtS) (Doose syndrome)
- Phenotype somewhat similar to LGS
- Myoclonic astatic seizures more prominent
- Genetic (rather than lesional) cause
- Onset 1.5 to 5 years
- Variable response to treatment
EEG
- Background slow 4-7Hz
- Slow spike wave during sleep usually not as prominent as in LGS
- Myoclonic
Infantile epileptic spasms syndrome (IESS) (West Syndrome)
Encompasses classic West Syndrome, but also those with spasms who do not meet all three criteria of Wests.
West Syndrome - Triad of:
o Infantile spasms
o Hypsarrhythmia on EEG
o Neurodevelopmental disorder
Onset between 3 months and 3 years
Aetiology
Multiple possible underlying aetiologies (>200), often structural e.g.
o Tuberous sclerosis
o Cortical dysplasia
o Tumour
o Stroke, hypoxic ischaemic encephalopathy
o Genetic Trisomy 21, many single gene disorders
Aetiology unknown in 30%
Clinical
Infantile spasms are the hallmark
o Flexion, extension or mixed type
o Flexor spasms
- Brief tonic contraction in flexion at hips and neck
- Tensing of the shoulders sometimes in abduction
- Jack-knife seizures
o Typically held for 1 sec (i.e. longer than a myoclonic seizure)
o Tend to occur in clusters that may last for several minutes
A proportion will go on to develop LGS
EEG
Hypsarrhythmia (Greek high or lofty)
High voltage, chaotic rhythm
Activated by sleep
o Suppressed in REM sleep
Modified Hypsarrhythmia
o When does not quite meet full definition
o One example would be when activity is only seen during sleep and the awake EEG is relatively normal
Spasms
o Complex slow wave followed by low voltage, rapid spikes
Management
Depends on underlying cause
ACTH
Corticosteroids
Vigabatrin
(Particularly effective if tuberous sclerosis is underlying cause)
Ketogenic diet
Levetiracetam
Benzos
VPA
Surgery
Dravet Syndrome
Previously known as Severe Myoclonic Epilepsy of Infancy
Clinical
Normal infant
Seizures from 6 months
Focal clonic/Hemiclonic or tonic-clonic seizures
Often associated with trigger:
o Fever (can initially appear like a typical benign febrile seizure)
o Infection
o Environmental heat/hot baths
o Immunisation
o Sunlight
o Pattern stimulation
o Exercise
o Excitment
Prolonged seizures/status epilepticus then develop
Frequent seizures over next 6/12
Other seizures by 1-4yrs
o Myoclonus
o Absence
o Focal seizures
o Tonic
o Atonic
Other features
o Developmental delay
o Crouching gait
o Speech deficits almost universal
o Parkinsonism can develop in later life
EEG
Initial interictal EEG is often normal
No characteristic features
Aetiology
80% have SCN1A mutation (sodium channel)
95% de-novo mutation
Type of mutation can correlate with severity of phenotype
o Truncating mutations generally worse
Can overlap with GEFS+ (which is also due to SCN1A mutation)
Treatment
Avoid triggers
o Aggressively treat fevers and infections
Avoid sodium channel blockers (given SCN1A encodes a Na Channel)
o It is unclear if this is absolute in adult patients. Some adult patients have been observed to have a good response to lamotrigine.
o Phenytoin should be avoided as a long term treatment but may be effective in status epilepticus

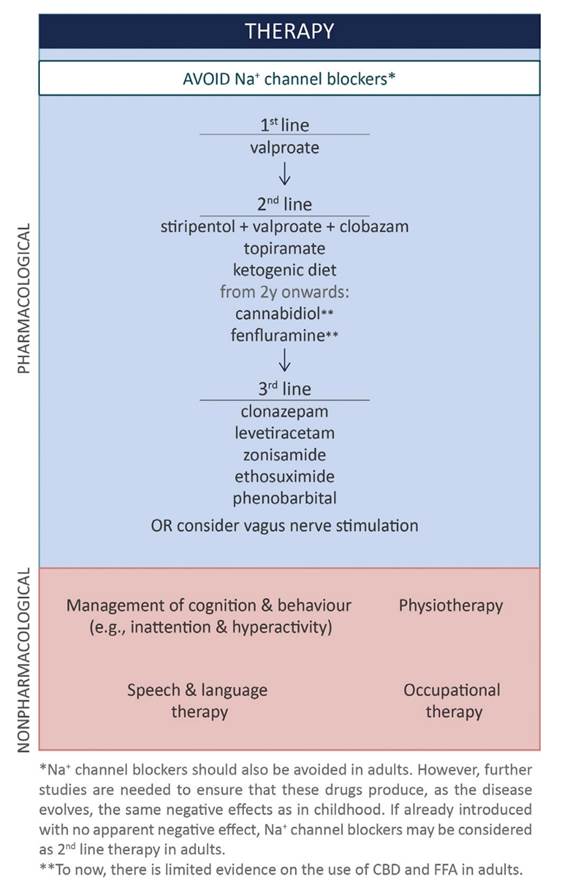
Stiripentol
o Reduces seizure frequency (13% in one study c.f. 51% with valproate)
o May be particularly effective at decreasing length/severity of status epilepticus
o Probably less useful in older patients
Canabidiol
o RCT demonstrated 41% reduction vs 16% in placebo group
o Can push up clobazam levels
Fenfluramine
o Old serotonergic drug, previously used as anti-obesity, was associated with valve disease in old trials
o 50-70% response rate vs ~7% in placebo
o Well tolerated in trials no evidence of valve disease
Ketogenic diet
o Some evidence of efficacy
o Less tolerated as patient ages
Behaviour management
o Methylphenidate used in some cases
Prognosis
15-20% mortality
Largely due to SUDEP
Developmental delay
References:
Guidance on Dravet Syndrome, Epilepsia open 2021
Progressive Myoclonic Epilepsies
Myoclonic
GTCS
Progressive neurological deficits
Causes
o Unverricht-Lundborg disease
o MERFF
o Lafora
o Neuronal ceroid lipofuscinosis
Progressive, dementing conditions
Neuronal ceroid lipofuscinosis
Lafora body disease
Mutation in Laforin gene
Diagnosis by detection of Lafora bodies in skin biopsies
Onset 6-19
GTCS and myoclonic seizures
Also focal seizures with preserved awareness and visual seizures
Condition progresses with severe ataxia, myoclonus and dementia
Death occurs 2-10 years after onset
Non-dementing
MERRF
See Mitochondrial disease topic
Variable age of onset
Unverricht-Lundborg disease (Baltic Myoclonus)
Cystatin B mutation
Onset age 7-16
Action myoclonus
Later GTCS
Ataxia
Can be worsened by phenytoin
MEAK
Myoclonic and Epilepsy with Ataxia and Potassium Chanel
Described by Austin group (2015)
Onset 5-15
Looks like JME to start with but then progresses to refractory myoclonus
Wheel-chair bound in late teens due to myoclonus
Cognition generally normal
De novo mutation in potassium channel
Familial Adult Myoclonic Epilepsy (FAME)
· Tremor and seizures (?focal or generlaised
· EEG not always abnormal usu generlaised
Developmental Epileptic Encephalopathy Spike and wave activation in sleep (DEE-SWAS/EE-SWAS) including Landau-Kleffner
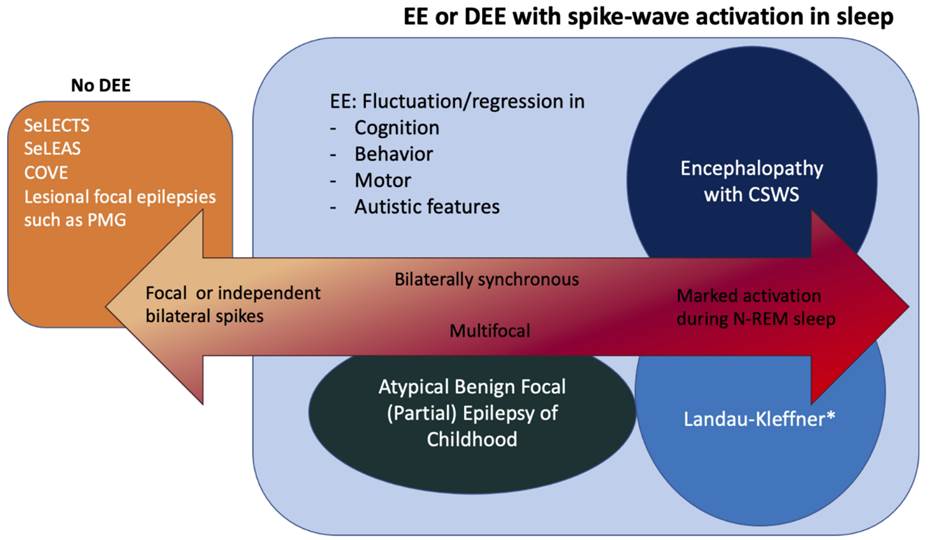
Normal development with isolated language delay
Speech regression with auditory agnosia
Behavioural difficulties
70% mild epilepsy
EEG
Continuous spike-wave in sleep (CSWS) for 85% of non-REM sleep
Concept of Developmental/Epileptic Encephalopathies
· The pattern in which brain reacts to an insult depends on the patients age
· Thus the concept of age- dependent epileptic encephalopathies
· The three main patterns are (all described in detail above):
o EIEE
o WEST Syndrome
o LGS
Genetic focal epilepsies
Sleep related hypermotor epilepsy (SHE)
Previously ADFLE
Familial Mesial Temporal Lobe Epilepsy (FMTLE)
Familial Focal Epilepsy with Variable Foci (FFEVF)
Epilepsy with Auditory Features (EAF)
Self-Limited Focal Epilepsies (SeLFEs)
Childhood occipital visual epilepsy (COVE)
- Previously called: Late onset benign occipital epilepsy, Gastaut syndrome, idiopathic childhood occipital epilepsy-Gastaut type
Photosensitive occipital lobe epilepsy (POLE)
- Rare 0.7% of childhood epilepsies
Idiopathic Focal Epilepsies
Self-Limited Epilepsy with Centrotemporal Spikes (SeLECTS)
(Benign Rolandic Epilepsy)
·
10-20% of childhood epilepsies
·
Onset 3-13yrs
·
Most common simple partial seizure involving the
face
·
Originate from perisylvian
(rolandic) sensorimotor cortex which represents face
and oropharynx
·
Motor activity in upper extremity can also occur
·
Ύ seizures occur at night or on awakening
·
50% have a tonic-clonic
seizure at some time.
·
EEG Centrotemporal sharp waves with distinctive
features
o
Biphasic, negative sharp peak followed by a
positive rounded component
o
Occur in 1-2% of asymptomatic children as well
·
Spontaneous remission in majority by age 13yrs
Self-Limited Epilepsy with Autonomic Seizures (SeLAS)
(Panayiotopoulos syndrome)
Specific epilepsy
syndromes Symptomatic/Lesional Focal Epilepsies
Mesial
Temporal Lobe Epilepsy with hippocampal sc
- Commonest
cause of adult epilepsy
- Frequently a
history of early cerebral insult
(febrile seizure
- Complex
partial seizures
- Characteristic
hippocampal sclerosis
- Auras common
- Visceral(epigastric),
gustatory, dysmnestic, affective
- Partial awareness
commonly preserved
- Dystonic
posturing of the contralateral upper limb
- Infrequent
secondary generalized seizures
- Prominent
motor arrest motionless stare
- Refractory to
medication, responds well to surgery
Hypothalamic
Hamartoma
Precocious puberty
Laughing seizures from infancy
Evolve into partial seizures
Focal Cortical Dysplasia
Blumcke Classification (2011)
|
Type |
|
|
|
|
I |
Focal cortical
dysplasia with abnormal cortical lamination |
|
|
|
II (Taylor type) |
Focal cortical dysplasia
with dysmorphic neurons |
A: Without balloon
cells B: with balloon
cells |
Radial-glial bands
on MRI |
|
|
|
|
|
|
III |
Architectural
distortion of the cortical layer |
A: in temporal lobe
with hippocampal atrophy B: adjacent to glial
or glioneuronal tumour C: Adjacent to
vascular malformation D: Adjacent to
other lesions acquired in early childhood |
|
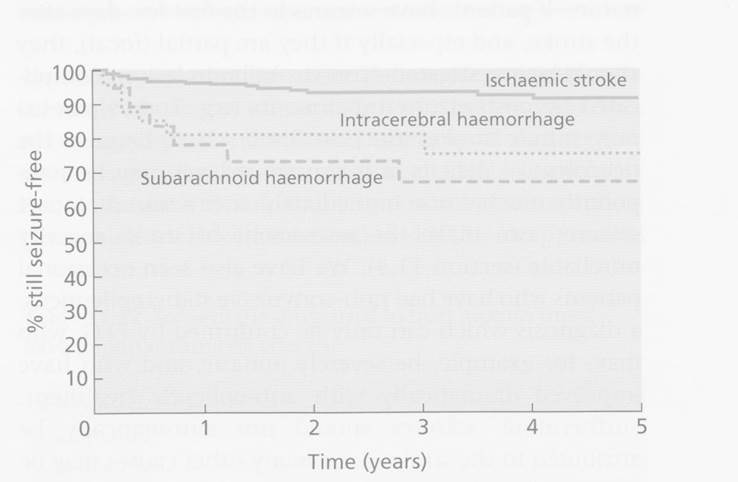
Post Stroke Seizures
·
5% of patients will have a seizure within first week (most in first
24hours)
o
More common in haemorrhagic and strokes affecting cortex
·
Later seizure risk
o
3-5% in first year and 1-2%/year thereafter
o
3% of all patients have >1 seizure and could be considered epileptic
o
This represents 20x increased risk compared to control population.
o
Risk of recurrent seizure after first seizure is 50%.
|
Gene |
Function |
|
Clinical Syndromes |
Benefit of testing |
|
SCN1A |
Sodium channel type 1alpha |
|
Dravet syndrome OR Genetic Epilepsy with Febrile
Seizures Plus (GEFS) OR Early infantile SCN1A encephalopathy
(rare) |
Diagnosis AED choice (sodium channel blockers
make things worse except lamotrigine).
VPA and Benzodiazepines good. Stiripentol
useful. |
|
SLC2A1 |
Mutations in GLUT1 |
Usually de novo mutations |
GLUT1 Encephalopathy Early-onset childhood absence
epilepsy Overlap with movement disorders |
Diagnosis Ketogenic diet |
|
PCDH19 |
Protocadherin 19 |
|
Female patients with clustered focal
seizures, (onset under age 3, often with intellectual disability. |
Counselling |
|
KCNQ2 |
|
|
Epileptic encephalopathy OR Benign epilepsy syndrome |
?Ezogabine in future |
|
KCNT1 |
Sodium activated potassium channel |
|
Autosomal dominant frontal lobe
epilepsy (ADNFLE often with psychiatric features and ID) OR Epilepsy in infants with migrating
seizure focus (EIMFS) poor prognosis |
?Quinidine |
|
?CHRN |
|
|
ADNFLE |
|
|
PRRT2 |
Binds to SNAP25 - |
|
Paroxysmal Kinesogenic
Dyskinesia (PKD) - overlaps with Benign familial infantile epilepsy |
Carbamazepine or oxcarbazepine might
be effective |
|
TSC1 and TSC2 |
|
|
Tuberous sclerosis |
Vigabatrin might be effective ?Rapamycin might be effective |
|
ALDH7A1 and PNPO |
|
|
Severe, early onset epilepsy |
Pyridoxine |
|
Experimental |
|
|
|
|
|
DEPDC5 |
DEP containing domain 5 |
|
Familial focal epilepsy with variable
foci |
|
|
GRIN2A |
NMDA receptor subunit epsilon1 |
|
Landau-Kleffner
syndrome Epilepsy aphasia disorders |
|
|
|
|
|
|
|
Familial Focal Epilepsy
First degree relatives of focal epilepsy patients have a 2.5x risk of epilepsy
Autosomal Dominant Sleep-related Hypermotor Epilepsy (ADSHE)
Previously Autosomal dominant frontal lobe epilepsy (ADFLE)
Onset in childhood
Seizures
o Brief tonic or hyperkinetic
o Tend to occur in clusters
o Occur during sleep shortly after falling asleep or before waking
o Auras common
o Often with preserved awareness during seizures
Treatment
o Carbamazepine
Prognosis
o Usually relatively benign
Aetiology
o CHRN Mutations (Nicotinic AChR)
o DEPDC5
o KCNT1
o Mutation has some correlation with severity of phenotype
Autosomal Dominant Epilepsy with Auditory Features
Seizures that originate in the lateral temporal lobe
Seizures:
o Prominent auditory aura
o Sometimes receptive aphasia
o Usually poorly formed sounds (ringing, buzzing)
o Sometimes voices
o Sometimes a sudden loss of sound
o Can have focal aware, FIAS and FBLTC
Aetiology:
o LGI1 in 30-50%
o RELN mutations (17%)
Familial Focal Epilepsy with Variable Foci (FFEVF)
Focal epilepsy has variable foci in different family members, but constant focus within individual
o E.g. Families with temoral, frontal, parietal and occipital lobe epilepsies
Variable age of onset and severity
May be co-morbid psychiatric conditions
Often lesion negative, however some cases associated with focal cortical dysplasia and subcortical grey matter heterotopia
Aetiology:
o DEPDC5 ~80% of families
- Protein is a member of the GATOR1 complex which inhibits the mTOR pathway
o NPRL2 and NPRL3 genes (encode different parts of GATOR1 pathway)
Familial mesial temporal lobe epilepsy
Defined by mesial temporal lobe epilepsy with strong family history (otherwise can look like sporadic MTLE)
Onset adolescence or early adulthood
Seizures:
o Focal aware seizures with prominent psychic or autonomic features
o Intense dιjΰ vu, dream like states, flashbacks, fear, epigastric sensations, flushing, olfactory sensations
o Sometimes FIAS
o Infrequent FBTC (often from sleep)
Aetiology
o Most cases unknown
o DEPDC5 in a small percentage of cases
o ~20% of cases of MTLE
Relatively benign and responsive to medication compared to other forms of MTLE
Other:
Familial neonatal epilepsy
Familial neonatal-infantile epilepsy
Familial infantile epilepsy